Answer:
Results are consistent with law of multiple proportions.
Step-by-step explanation:
Dalton's law of multiple proportions states that the elements can be combined to form more than one compound and do so in such a way that if we keep the quantity of one element as fixed, the quantity of the other element is added in such a way that they are obtained integer proportions.
Molar mass of oxygen is 16g per mole, molar mass of phosphorus is 31g per mole.
In
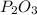 the ratio between P and O is
the ratio between P and O is
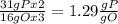 , that is consistent with the experimental result.
, that is consistent with the experimental result.
and for
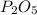 the ratio between P and O is
the ratio between P and O is
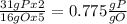 that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.
that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.