Answer:
132 ℃ is the final temperature.
Step-by-step explanation:
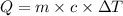
where ∆T =final T- initial T
Q is the heat energy in calories = 146cal
c is the specific heat capacity
(for Lead (0.128 J/(g℃)) or (0.0305 cal/(g℃)
m is the mass of lead = 57g
Initial temperature is 48℃
Plugging in the values
146cal = 57g × 0.0305 cal/(g℃) × (final T - 48℃)
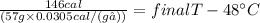
83.98℃ = final T - 48℃
So,
Final T = 83.98℃ + 48℃
Final T = 131.98℃ = 132℃ (Answer)