Answer:
A. Attractive forces called hydrogen bonds
Step-by-step explanation:
Liquid water contains water molecules. The attractive forces between the water molecules is called HYDROGEN BONDING.
Hydrogen bonding is the intermolecular forces which exists between h atom and the highly electronegative atoms like N, O, F.
For example
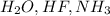
It is a polar covalent bond.
It has a strong intermolecular forces.
Its strength is more than that of the London dispersion forces
It is also called as a dipole –dipole forces.
Dipole –dipole forces is the forces which exist between the polar molecules.
The electronegative values of H is 2.1
F is 4.0
O is 3.5 and
N is 3.0
∆E value of HF Hydrogen bond is 4.0 - 2.1 = 1.9
∆E value of
 Hydrogen bond is 3.5 - 2.1 = 1.4
Hydrogen bond is 3.5 - 2.1 = 1.4
∆E value of
 Hydrogen bond is 3.0 - 2.1 = 0.9
Hydrogen bond is 3.0 - 2.1 = 0.9
∆E value between 0.5 to 1.7 is a polar covalent bond
Hence Hydrogen bond is a bond between polar molecules and also comes under the category of dipole-dipole forces.