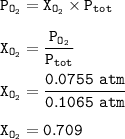P tot = 0.1065 atm
The mole fraction of oxygen in the mixture : 0.709
Further explanation
Given
10 L flask
1.013 g O₂
572 g CO₂
T = 18 °C = 291 K
Required
P O₂ and P CO₂
Solution
Dalton Law's of partila pressure
P tot = P₁ + P₂ + ..Pₙ
From ideal gas Law :
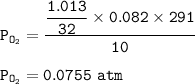
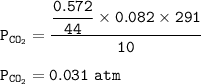
P tot = P O₂ + P CO₂
P tot = 0.0755 + 0.031
P tot = 0.1065 atm
The mole fraction of O₂ :