The volume did not change, it remained at 20 ml
Further explanation
Given
20 ml a sample gas at STP(273 K, 1 atm)
T₂=546 K
P₂=2 atm
Required
The volume
Solution
Combined gas Law :
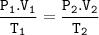
Input the value :
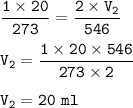
The volume does not change because the pressure and temperature are increased by the same ratio as the initial conditions (to 2x)