Answer:
total number of electron in 1 litter is 3.34 ×
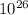 electron
electron
Step-by-step explanation:
given data
mass per mole = 18 g/mol
no of electron = 10
to find out
how many electron in 1 liter of water
solution
we know molecules per gram mole is 6.02 ×
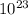 molecules
molecules
no of moles is 1
so
total number of electron in water is = no of electron ×molecules per gram mole × no of moles
total number of electron in water is = 10 × 6.02 ×
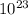 × 1
× 1
total number of electron in water is = 6.02×
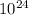 electron
electron
and
we know
mass = density × volume ..........1
here we know density of water is 1000 kg/m
and volume = 1 litter = 1 ×
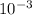 m³
m³
mass of 1 litter = 1000 × 1 ×
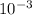
mass = 1000 g
so
total number of electron in 1 litter = mass of 1 litter ×

total number of electron in 1 litter = 1000 ×

total number of electron in 1 litter is 3.34 ×
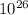 electron
electron