Answer:
For diatomic oxygen:V=539.06 m/s
For carbon dia oxide:V=459.71 m/s
For dia atomic hydrogen:V=2156.25 m/s
Step-by-step explanation:
As we know that
Root mean square velocity V

Where
R is the gas constant
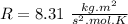
T is the temperature (K).
M is the molecular weight.
For diatomic oxygen:
M=32 g/mol
T=273+100 = 373 K
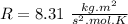

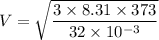
V=539.06 m/s
For carbon dia oxide
M=44 g/mol
T=273+100 = 373 K
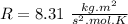

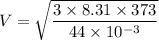
V=459.71 m/s
For dia atomic hydrogen:
M= 2 g/mol
T=273+100 = 373 K
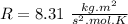

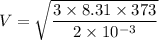
V=2156.25 m/s