Answer:
You need 0.8 ml of 5M stock solution and you have to add 9.2 ml of water.
Step-by-step explanation:
Protocol solution (1X): 100 mM=0.1M
4X: 0.4M
The concentration of a solution is inversely proportional to the volume of a solution, so:
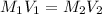
where:
M1= 5M stock solution
V1= amount of solution we need to collect
M2=4X solution
V2= 10 ml (volumen of 4X solution)
Therefore:
5M×V1=0.4M×10ml
V1={0.4M}{5M}10ml=0.8ml
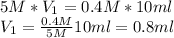
To make a 10 ml solution we have to add 9.2 ml of water because V2 es 10 ml.