Answer:
Volume of water added = 2.0 L
Step-by-step explanation:
Initial pH of the solution = 2.7
[H^+] concentration in 2 L solution of sulfuric acid,
![pH = -log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/college/gny7xuakxqc45obx08p22znzokdd3vdn81.png)
![[H^+] = 10^(-pH)\ M](https://img.qammunity.org/2020/formulas/chemistry/college/nq9mpl3lj791w95o70klanm9wv8kf5vaeb.png)
![[H^+] = 10^(-2.7) = 0.001995\ M = 0.002\ M](https://img.qammunity.org/2020/formulas/chemistry/college/c8fbhuvth17g1hdw7gbrey00dpg9paf5fx.png)
Final pH of the solution = 3
Final [H^+] concentration of sulfuric acid,
![[H^+] = 10^(-pH)\ M](https://img.qammunity.org/2020/formulas/chemistry/college/nq9mpl3lj791w95o70klanm9wv8kf5vaeb.png)
![[H^+] = 10^(-3) = 0.001\ M](https://img.qammunity.org/2020/formulas/chemistry/college/pom92bbtq6zckq9vs1tn58vdm6mbvylh6z.png)
Now,

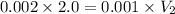
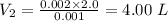
Volume added = Final volume - Initial volume
= 4.0 - 2.0 = 2.0 L