Answer:
Incomplete precipitation of barium sulfate
Step-by-step explanation:
The student has precipitated and digested the barium sulfate on his/her side. But on the addition of
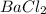 in the solution, the solution become cloudy. This happened because incomplete precipitation of barium sulfate by the student. When
in the solution, the solution become cloudy. This happened because incomplete precipitation of barium sulfate by the student. When
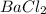 is added, there are still sulfate ions present in the solution with combines with
is added, there are still sulfate ions present in the solution with combines with
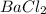 and forms
and forms
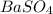 and the formation of this precipitate makes the solution cloudy.
and the formation of this precipitate makes the solution cloudy.