Answer:
0.075 moles of EDTA are left.
Step-by-step explanation:
To solve this problem we need to use the definition of Molar concentration (M):
M=

With that equation in mind, in the problem we're given two out of the three factors: Concentration and Volume. We're asked to calculate the remaining factor.
Before we calculate we convert 150 mL to L, so we divide 150 by 1000.
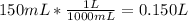
Lastly we calculate the number of moles of EDTA left:
0.5 M * 0.150 L = 0.075 moles.