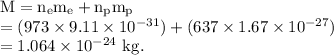Answer:
Number of electrons in the system = 973.
Total mass of the system =
Step-by-step explanation:
Assumptions:
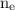 = number of electrons in the system.
= number of electrons in the system.
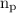 = number of protons in the system.
= number of protons in the system.
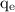 = charge on an electron =
= charge on an electron =
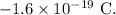
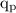 = charge on a proton =
= charge on a proton =
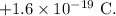
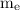 = mass of an electron =
= mass of an electron =
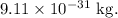
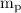 = mass of a proton =
= mass of a proton =
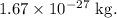
Given:
- Total number of particles in the system, N = 1610.
- Net charge on the system, q =
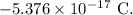
Since, the system is comprised of electrons and protons only, therefore,
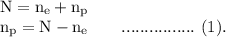
The net charge on the system can be written in terms of charges on electrons and protons as

Putting the value of (2) in (1), we get,
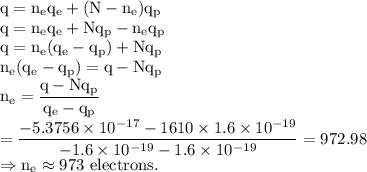
It is the number of electrons in the system.
Therefore, the number of protons is given by
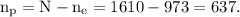
The total mass of the system is given by