Answer:
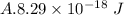
Step-by-step explanation:
Given that:
p = magnitude of charge on a proton =
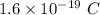
k = Boltzmann constant =
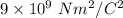
r = distance between the two carbon nuclei = 1.00 nm =
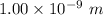
Since a carbon nucleus contains 6 protons.
So, charge on a carbon nucleus is
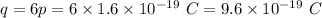
We know that the electric potential energy between two charges q and Q separated by a distance r is given by:
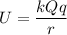
So, the potential energy between the two nuclei of carbon is as below:
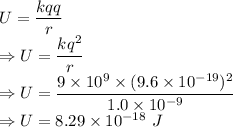
Hence, the energy stored between two nuclei of carbon is
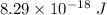 .
.