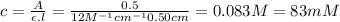Answer:
The concentration of the copper sulfate solution is 83 mM.
Step-by-step explanation:
The absorbance of a copper sulfate solution can be calculated using Beer-Lambert Law:
A = ε . c . l
where
ε is the extinction coefficient of copper sulfate (ε = 12 M⁻¹.cm⁻¹)
c is its molar concentration (what we are looking for)
l is the pathlength (0.50 cm)
We can use this expression to find the molarity of this solution: