Answer:
8 drops of 1.00 M NaOH will be needed.
Step-by-step explanation:
Concentration of
![[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xxde7ud270ullhgkdtd9pogar35adf8qg0.png) in bleach solution = 0.02 M
in bleach solution = 0.02 M
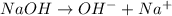
![NaOH=[OH^-]=0.02M](https://img.qammunity.org/2020/formulas/chemistry/college/up4tif8wq32wpbl74b20qemi2j2pv4cb54.png)
Concentration of bleach solution we want ,
 = 0.02 M
= 0.02 M
Volume of the bleach solution,
 = 20 ml
= 20 ml
Concentration of NaOH solution,
 = 1.00 M
= 1.00 M
Volume of the NaOH solution required ,
 = ?
= ?

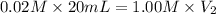
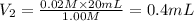
1 mL = 20 drops
0.4 mL = 0.4 × 20 drops = 8 drops
8 drops of 1.00 M NaOH will be needed.