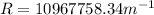Answer:
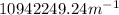
Step-by-step explanation:
Rydberg's formula is used to describe the wavelengths of the spectral lines of chemical elements similar to hydrogen, that is, with only one electron being affected by the effective nuclear charge. In this formula we can find the rydberg constant, knowing the wavelength emitted in the transcision between two energy states, we can have a value of the constant.
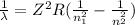
Where
 it is the wavelength of the light emitted, R is the Rydberg constant, Z is the atomic number of the element and
it is the wavelength of the light emitted, R is the Rydberg constant, Z is the atomic number of the element and
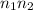 are the states where
are the states where
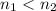 .
.
In this case we have Z=1 for hydrogen, solving for R:
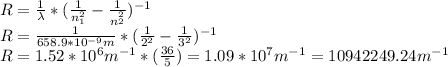
This value is quite close to the theoretical value of the constant