Answer:
Without doing any calculations it is possible to determine that silver chloride is more soluble than ACD, and silver chloride is less soluble than ___.
It is not possible to determine whether silver chloride is more or less soluble than B by simply comparing Ksp values.
Step-by-step explanation:
The Lead (Pb) is a heavy metal and Zinc (Zn) is a transition metal, both combine with the anion phosphate produces heavy complexes very insoluble.
The silver cation (Ag+) is an acid cation which in basic conditions rapidly precipitates as AgOH which is highly insoluble and unstable.
The FeCO3 is a complex with a solubility relatively close between AgCl. The Kps of these two are
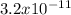 and
and
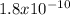 respectively at room temperature.
respectively at room temperature.