Step-by-step explanation:
The hybridization of the carbon in which it forms 120° with the other atoms is
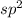 .
.
In
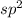 hybridized carbon, it forms a geometry of trigonal planar which has angle of 120°. This corresponds to doubly bonded carbon.
hybridized carbon, it forms a geometry of trigonal planar which has angle of 120°. This corresponds to doubly bonded carbon.
To draw:
One carbon-carbon double bond in 4 membered carbon chain.
The structure is shown in image below.