Answer:
1.69224581396 Kg/L
Explanation:
We are given the measure of the block as 4.45 cm × 3.35 cm × 6.15 cm.
Volume of block = 4.45 cm × 3.35 cm × 6.15 cm = 91.681125 cm cube = 91.681125 × 0.001 L = 0.091681125 L
We did the above step to convert the volume of block into Liter.
Mass of block is given as 155.147 gram = 155.147 × 0.001 kg = 0.155147 kg
We converted the mass of block into kilograms because we need density in Kg/L.
Density is defined as mass per unit volume
Density =
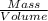
=
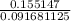 ]
]
= 1.69224581396 Kg/L