Answer:
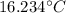
Step-by-step explanation:
Given
Mass of object (m)=6 kg
falling height(h)=10 m
mass of water(
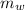 )=600 gm
)=600 gm
temperature of water =15
specific heat of water
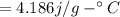
Let T be the Final Temperature of water
Here Object Potential Energy is converted into Heat energy which will be absorbed by water
Potential Energy(P.E.)
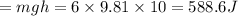
Heat supplied
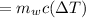
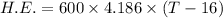
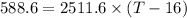
T-16=0.234
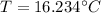
This is not an efficient way of heating water as there is only
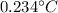 increase in temperature.
increase in temperature.