Answer: enthalpy of reaction.
Step-by-step explanation:
Temperature of the gas is defined as the degree of hotness or coldness of a body. It is expressed in units like
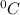 and
and
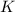
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
Free energy is the amount of energy that can be converted into useful work.
Enthalpy of the reaction is the difference between the energy of products and the energy of reactants. it is either the heat released or absorbed during the reaction. It is either positive or negative.