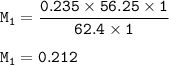The molarity of the HNO₃ solution : 0.212 M
Further explanation
Given
62.4 mL HNO₃
56.25 mL of a 0.235 M KOH
Required
Molarity HNO₃
Solution
Titration formula
M₁V₁n₁=M₂V₂n₂
1= HNO₃, 2 = KOH
n = acid base valence (amount of H⁺/OH⁻ released,for HNO₃ and KOH = 1)
Input the value :
M₁ x 62.4 x 1 = 0.235 x 56.25 x 1