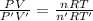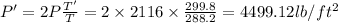Answer:
The inside Pressure of the tank is
Solution:
As per the question:
Volume of tank,
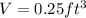
The capacity of tank,
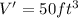
Temperature, T' =
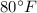 = 299.8 K
= 299.8 K
Temperature, T =
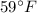 = 288.2 K
= 288.2 K
Now, from the eqn:
PV = nRT (1)
Volume of the gas in the container is constant.
V = V'
Similarly,
P'V' = n'RT' (2)
Also,
The amount of gas is double of the first case in the cylinder then:
n' = 2n
![\]frac{n'}{n} = 2](https://img.qammunity.org/2020/formulas/physics/college/j4soa314cup2szyq0gjhc7cyj1y160fhjb.png)
where
n and n' are the no. of moles
Now, from eqn (1) and (2):