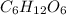Answer: The molecular formula of glucose is
Step-by-step explanation:
We are given:
Empirical formula of the compound =
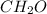
Empirical mass of the compound =
![[(1* 12)+(2* 1)+(1* 16)]=30g/mol](https://img.qammunity.org/2020/formulas/chemistry/college/u1qhyd04wca1pjze6gvhoypbor1hskf0b5.png)
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is:
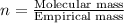
We are given:
Mass of molecular formula = 180.12 g/mol
Mass of empirical formula = 30 g/mol
Putting values in above equation, we get:
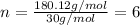
Multiplying this valency by the subscript of every element of empirical formula, we get:
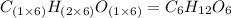
Hence, the molecular formula of glucose is