Answer:
You need 8,324 g of CaCl₂ yo make this solution
Step-by-step explanation:
Molarity is a way to express concentration in a solution, in units of moles of solute per liter of solution.
To know the grams of CaCl₂ it is necessary to know, first, the moles of this substance with the desired volume and concentration , thus:
0,1500 L ×
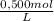 = 0,075 CaCl₂ moles
= 0,075 CaCl₂ moles
Now, with the molar mass of CaCl₂ you will obtain the necessary grams, thus:
0,075 CaCl₂ moles ×
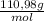 = 8,324 g of CaCl₂
= 8,324 g of CaCl₂
So, you need 8,324 g of CaCl₂ to make 150,0 mL of a 0,500M solution
I hope it helps!