Answer:
a) 3.39 × 10²³ atoms
b) 6.04 × 10⁻²¹ J
c) 1349.35 m/s
Step-by-step explanation:
Given:
Diameter of the balloon, d = 29.6 cm = 0.296 m
Temperature, T = 19.0° C = 19 + 273 = 292 K
Pressure, P = 1.00 atm = 1.013 × 10⁵ Pa
Volume of the balloon =
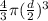
or
Volume of the balloon =
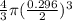
or
Volume of the balloon, V = 0.0135 m³
Now,
From the relation,
PV = nRT
where,
n is the number of moles
R is the ideal gas constant = 8.314 kg⋅m²/s²⋅K⋅mol
on substituting the respective values, we get
1.013 × 10⁵ × 0.0135 = n × 8.314 × 292
or
n = 0.563
1 mol = 6.022 × 10²³ atoms
Thus,
0.563 moles will have = 0.563 × 6.022 × 10²³ atoms = 3.39 × 10²³ atoms
b) Average kinetic energy =
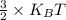
where,
Boltzmann constant,
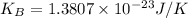
Average kinetic energy =
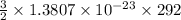
or
Average kinetic energy = 6.04 × 10⁻²¹ J
c) rms speed =
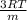
where, m is the molar mass of the Helium = 0.004 Kg
or
rms speed =
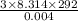
or
rms speed = 1349.35 m/s