Answer:
Part A : n = 1.77 moles
Part B : λ =

Part C : n = 0.154 moles
Step-by-step explanation:
Part A
The problem gives you the equation for molarity M:
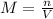
n is the number of moles of solute and V is the volume
Then the problem gives you the molarity of a substance
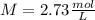 and the volume V = 0.650L, so you need to solve the equation for n:
and the volume V = 0.650L, so you need to solve the equation for n:
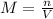
as the V is dividing it passes to multiply the M:
n = M*V
and you should replace the values:

n = 1.77 moles
Part B
This time you have to solve the equation E = hcλ for λ that is the unknown information, so you have:
E = hcλ
h and c are multiplying so they pass to divide the E:
λ =
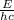
and replacing the values:
λ =
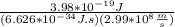
λ =

PartC
In this part the problem gives you the equation PV=nRT and the first thing you should do is to verify that all the quantities are in consistent units so:
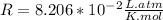 so you need to convert the pressure to atmospheres and convert the volume to liters.
so you need to convert the pressure to atmospheres and convert the volume to liters.
- Convert the pressure to atmospheres:
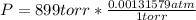
P = 1.18 atm
- Convert the volume to liters:
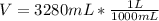
V = 3.28L
To find the number of moles n, you should solve the equation for n:
Pv = nRT
As R and T are multiplying the n, they pass to divide to the other side of the equation:
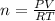
And finally you should replace the values:
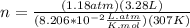
n = 0.154 moles