Answer:
Look picture
Step-by-step explanation:
The empirical formula is obtained with average of each atom by mass over Atomic weight, thus:
47,5% S ÷ 32,045 g/mol = 1,48 mol S
52,5% Cl ÷ 35,45 g/mol = 1,48 mol Cl
Thus, empirical formula is:
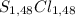 ≡ SCl.
≡ SCl.
The Lewis structure for this SCl molecule is in the picture. You can see one unpaired electron in S. These unpaired electrons are very unstable doing SCl an improbable molecule.
The more pausible structure with the same S:Cl ratio is S₂Cl₂ (Look picture). In this molecule you don't have unpaired electrons doing this compound more stable (In fact, exist, and its name is Disulfur dichloride)
I hope it helps!