Answer:
For a: The amount of heat produced for given amount of glucose is -283.05 kJ
For b: The amount of heat produced for given amount of glucose is -67648.9 Cal
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of glucose = 18.1 g
Molar mass of glucose = 180.16 g/mol
Putting values in above equation, we get:
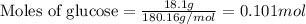
The given chemical reaction follows:
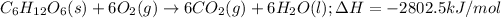
By Stoichiometry of the reaction:
When 1 mole of glucose is reacted, the amount of heat released is 2802.5 kJ
So, when 0.101 moles of glucose is reacted, the amount of heat released is
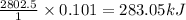
Hence, the amount of heat produced for given amount of glucose is -283.05 kJ
To convert the heat produced in kilo joules to calories, we use the conversion factor:
1 kJ = 239 Cal
So,
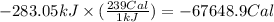
Hence, the amount of heat produced for given amount of glucose is -67648.9 Cal