Step-by-step explanation:
As per the given data, at a higher temperature, at
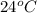 , the solution will occupy a larger volume than at
, the solution will occupy a larger volume than at
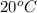 .
.
Since, density is mass divided by volume and it will decrease at higher temperature.
Also, concentration is number of moles divided by volume and it decreases at higher temperature.
At
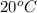 , density of water=0.9982071 g/ml
, density of water=0.9982071 g/ml
Therefore,
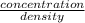 will be calculated as follows.
will be calculated as follows.
=
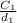
=
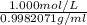
= 1.0017961 mol/g
At
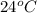 , density of water = 0.9972995 g/ml
, density of water = 0.9972995 g/ml
Since,
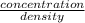 =
=
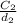
=
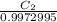
Also,
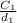 =
=
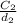
so, 1.0017961 mol/g =
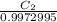
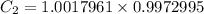
= 0.9990907 mol/L
Therefore, in 500 ml, concentration of
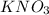 present is calculated as follows.
present is calculated as follows.
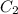 =
=
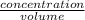
0.9990907 mol/L =
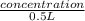
concentration = 0.49954537 mol
Hence, mass (m'') =
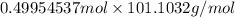 = 50.5056 g (as molar mass of
= 50.5056 g (as molar mass of
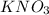 = 101.1032 g/mol).
= 101.1032 g/mol).
Any object displaces air, so the apparent mass is somewhat reduced, which requires buoyancy correction.
Hence, using Buoyancy correction as follows,
m =
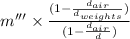
where,
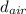 = density of air = 0.0012 g/ml
= density of air = 0.0012 g/ml
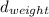 = density of callibration weights = 8.0g/ml
= density of callibration weights = 8.0g/ml
d = density of weighed object
Hence, the true mass will be calculated as follows.
True mass(m) =
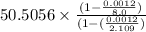
true mass(m) = 50.5268 g
= 50.53 g (approx)
Thus, we can conclude that 50.53 g apparent mass of
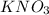 needs to be measured.
needs to be measured.