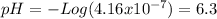Answer:
(a) pH = 4.56 is acidic
(b) pH = 10.4 is basic
(c) [OH-] = 2.4x10-8, thus
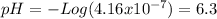 is acidic
is acidic
Step-by-step explanation:
The pH is the mesure of the acidity or bacisity of a solution. It indicates the concentration of protons in a solution and it is define as:
![pH = -Log ([H^(+)])](https://img.qammunity.org/2020/formulas/chemistry/college/t6s47t005ppu1e9e2czzgt6m5uxnp890uj.png)
The scale of pH goes from 1 to 14, being 1 the most acid condition and 14 de most basic condition. Also a pH = 7 is a neutral condition.
Therefore, if the pH is: 1 ≤ pH < 7 the solution will be acidic and if the pH is 7 < pH ≤ 14 the solution will be basic.
To answer (c) it is also necessary to consider the water autoionization to calculate the protons concentration as shown bellow
![K_(w) =[H^(+) ][OH^(-)]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/college/xtnf0gn18rehlak9tcxr5t7tr5p1kukt9d.png)
![[H^(+) ]=(10^(-14))/([OH^(-)])](https://img.qammunity.org/2020/formulas/chemistry/college/e6cf7mmtm48f3bz5wv5pxa7fdbx193dd1r.png)
For (c) [OH-] = 2.4x10-8
![[H^(+) ]=(10^(-14))/(2.4x10^(-8))=4.16x10^(-7)](https://img.qammunity.org/2020/formulas/chemistry/college/k3krg9d9r3xdllyxb53vq86zyru8a8kitr.png)
And using the definition of pH