Answer:
0.0375 moles
Step-by-step explanation:
Given that:
Molar mass of monopotassium phosphate = 136.09 g/mol
Given that volume = 250.0 mL
Also,
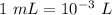
So, Volume = 250 / 1000 L = 0.25 L
Molarity = 0.15 M
Considering:

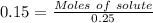
Thus, moles of monopotassium phosphate needed = 0.0375 moles