Answer:
W = 289.70 kg
Step-by-step explanation:
Given data:
Pressure in tank = 23 atm
Altitude 1000 ft
Air temperature in tank T = 700 F
Volume of tank = 800 ft^3 = 22.654 m^3
from ideal gas equation we have
PV =n RT
Therefore number of mole inside the tank is
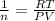
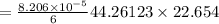
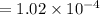
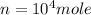
we know that 1 mole of air weight is 28.97 g
therefore, tank air weight is
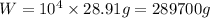
W = 289.70 kg