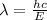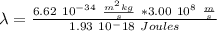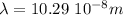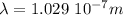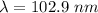Answer:
The wavelength required is 102.9 nm.
Step-by-step explanation:
The energy levels for the hydrogen atom are
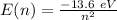
So, for a transition from the first level to the third level we got
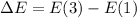
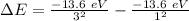
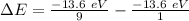
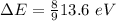
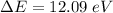
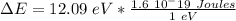
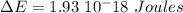
So we need a photon with this energy.
The energy of a photon its given by
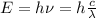
So, the wavelength will be