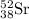Answer:
For a: The isotopic representation of iodine is
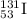
For b: The isotopic representation of cesium is
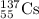
For c: The isotopic representation of strontium is
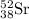
Step-by-step explanation:
The isotopic representation of an atom is:
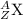
where,
Z = Atomic number of the atom
A = Mass number of the atom
X = Symbol of the atom
We are given:
Number of neutrons = 78
Atomic number of iodine = 53 = Number of protons
Mass number = 53 + 78 = 131
Thus, the isotopic representation of iodine is
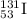
We are given:
Number of neutrons = 82
Atomic number of cesium = 55 = Number of protons
Mass number = 55 + 82 = 137
Thus, the isotopic representation of cesium is
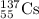
We are given:
Number of neutrons = 52
Atomic number of strontium = 38 = Number of protons
Mass number = 38 + 52 = 90
Thus, the isotopic representation of strontium is