Answer: The mass of carbon and hydrogen in the sample is 0.1087 g and 0.0066 g respectively and the percentage composition of carbon and hydrogen in the sample is 94.27 % and 5.72 % respectively.
Step-by-step explanation:
The chemical equation for the combustion of hydrocarbon having carbon and hydrogen follows:
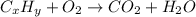
where, 'x' and 'y' are the subscripts of carbon and hydrogen respectively.
We are given:
Mass of
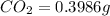
Mass of
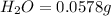
Mass of sample = 0.1153 g
We know that:
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
- For calculating the mass of carbon:
In 44g of carbon dioxide, 12 g of carbon is contained.
So, in 0.3986 g of carbon dioxide,
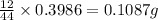 of carbon will be contained.
of carbon will be contained.
- For calculating the mass of hydrogen:
In 18g of water, 2 g of hydrogen is contained.
So, in 0.0578 g of water,
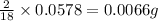 of hydrogen will be contained.
of hydrogen will be contained.
To calculate the percentage composition of a substance in sample, we use the equation:
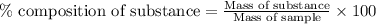 ......(1)
......(1)
Mass of sample = 0.1153 g
Mass of carbon = 0.1087 g
Putting values in equation 1, we get:
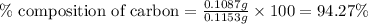
Mass of sample = 0.1153 g
Mass of hydrogen = 0.0066 g
Putting values in equation 1, we get:
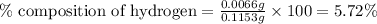
Hence, the mass of carbon and hydrogen in the sample is 0.1087 g and 0.0066 g respectively and the percentage composition of carbon and hydrogen in the sample is 94.27 % and 5.72 % respectively.