Answer:
Volume of HCl required = 28.4 mL
Step-by-step explanation:
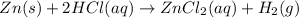
Mass of zinc ore = 4.65 g
% of zinc in zinc ore = 50 %
So, mass of zinc in zinc ore = 0.50 × 4.65 = 2.325 g
No. of moles of Zn =
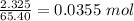
So, as per the reaction coefficient,
1 mol of zinc reacts with 2 mol of HCl
0.0355 mol of zinc reacts with
= 0.0355 × 2 = 0.071 mol of HCl
Molarity of HCl = 2.50 M
Volume of HCl =
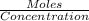
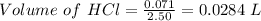
1 L = 1000 mL
0.0284 L = 1000 × 0.0284 = 28.4 mL