Answer:
0.152 moles
Step-by-step explanation:
Given that:
The mass of the oxygen gas produced = 4.87 g
Also, The molar mass of oxygen gas,
 =
=
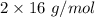 = 32 g/mol
= 32 g/mol
The formula for the calculation of moles is shown below:

Thus,
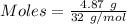
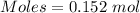
Both given values and the answer is in 3 significant digits.