Answer:
The hydroxide ion concentration is 0.003981 M.
The hydronium ion concentration is
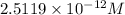 .
.
Step-by-step explanation:
The pOH of an aqueous solution at 25°C = 2.40
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
![2.40=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/college/tft1pxbv9xaf431tpcolntmzbcynjfskhq.png)
![[OH^-]=0.003981 M](https://img.qammunity.org/2020/formulas/chemistry/college/bhz885viq3ioby7uul2b8o3soqzada4zs0.png)
The hydroxide ion concentration is 0.003981 M.
The pH of an aqueous solution at 25°C = ?
The relationship between pH and pOH :
pH + pOH = 14
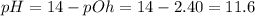
![pH=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/f390cegazdnm7uy3e4lyqajx4gquacwg62.png)
![11.6=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/83klu5zvn29ju0xwyfpz98e2uct6xer30y.png)
![[H_3O+]=2.5119* 10^(-12) M](https://img.qammunity.org/2020/formulas/chemistry/college/cditu72g6ghqse3rhk0ixp19i9mzv7hwqw.png)
The hydronium ion concentration is
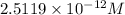 .
.