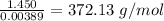Answer:
Molecular weight of the compound = 372.13 g/mol
Step-by-step explanation:
Depression in freezing point is related with molality of the solution as:
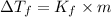
Where,
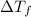 = Depression in freezing point
= Depression in freezing point
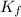 = Molal depression constant
= Molal depression constant
m = Molality
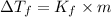
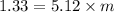
m = 0.26
Molality =
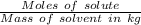
Mass of solvent (toluene) = 15.0 g = 0.015 kg
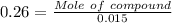
Moles of compound = 0.015 × 0.26 = 0.00389 mol
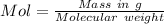
Mass of the compound = 1.450 g
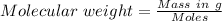
Molecular weight =