Answer:
The correct order is: Fluorine; Oxygen; Nitrogen; Carbon; Hydrogen; Sodium.
Step-by-step explanation:
The electronegativity in the periodic table increases to the right in a period and up in a group. We can figure out the electronegativity of each element according to its electron configuration:
Carbon: [He]
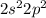
Fluorine: [He]
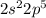
Hydrogen:
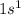
Nitrogen: [He]
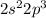
Oxygen: [He]
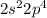
Sodium: [Ne]
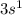
The period of a chemical element is given by the last energy level of electron configuration.
The group of a chemical element is given by the amount of electrons in the last energy level of electron configuration.
Therefore,
Carbon: Period 2 Group 4
Fluorine: Period 2 Group 7
Hydrogen: Period 1 Group 1
Nitrogen: Period 2 Group 5
Oxygen: Period 2 Group 6
Sodium: Period 3 Group 1
Electronegativity increases when the number of the group increases.
Electronegativity increases when the number of the period decreases.
In conclusion, Fluorine has the greatest number of group and Sodium has the lowest number of group (also it has a greater number of period than hydrogen, so it is less electronegative than hydrogen)
F>O>N>C>H>Na