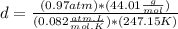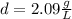Answer:
E.
Step-by-step explanation:
From the ideal gasses equation we have:
PV=nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
The number of moles is also expressed as:
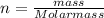
If replacing this in the ideal gasses equation we have:
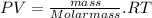
If we pass V to divide, we have:
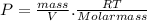
And the density d =
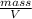 , so replacing, we have:
, so replacing, we have:
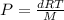
Solving for d, we have:
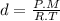
Now we have to be sure that we have the correct units, so we need to convert the units for pressure and temperature:
-Convert P=98kPa to atm
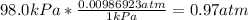
-Convert T=-25.2°C to K
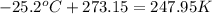
Finally we can replace the values in the equation: