Answer:
V of Sulfur tetrafluoride is 17.2 L
Step-by-step explanation:
Given data;
T = -6°C = 267K [1° C = 273 K]
n = 786 mmol of SF4 which is 0.786 mol
P = 1 atm
from ideal gas law we have
PV = nRT
where n is mole, R is gas constant, V is volume
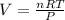
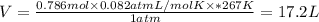
V of Sulfur tetrafluoride is 17.2 L