Answer:
The concentration is 50,8 % w/v and radio strengths = 1,96.
Step-by-step explanation:
Phenobarbital sodium is a medication that could treat insomnia, for example.
2,0 M of Phenobarbital sodium means 2 moles in 1L.
The concentration units in this case are %w/v that means 1g in 100 mL and ratio strengths that means 1g in r mL. Thus, 2 moles must be converted in grams with molar weight -254 g/mole- and liters to mililiters -1 L are 1000mL-. So:
2 moles ×
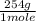 = 508 g of Phenobarbital sodium.
= 508 g of Phenobarbital sodium.
1 L ×
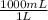 = 1000 mL of solution
= 1000 mL of solution
Thus, % w/v is:
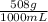 × 100 = 50,8 % w/v
× 100 = 50,8 % w/v
And radio strengths:
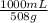 = 1,96. Thus, you have 1 g in 1,96 mL
= 1,96. Thus, you have 1 g in 1,96 mL
I hope it helps!