Step-by-step explanation:
The given data is as follows.
Mass of antimony = 19.75 g
Molar mass of Sb = 121.76 g/mol
Therefore, calculate number of moles of Sb as follows.
Moles of Sb =

=
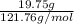
= 0.162 mol
Mass of oxygen given is 6.5 g and molar mass of oxygen is 16 g/mol. Hence, moles of oxygen will be calculated as follows.
Moles of oxygen =

=
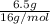
= 0.406 mol
Hence, ratio of moles of Sb and O will be as follows
Sb : O
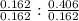
1 : 2.5
We multiply both the ratio by 2 in order to get a whole number. Therefore, the ratio will be 2 : 5.
Thus, we can conclude that the empirical formula of the given oxide is
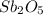 .
.