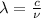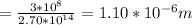Answer:
a)
3.43*10^{14} Hz
8.75*10^{-7} m
b)
2.70*10^{14} Hz
1.10*10^{-6} m
Step-by-step explanation:
GIVEN DATA:
a)
i)we know that
E = h\\u
where E is energy
h = plank's constant = 6.625* 10^{-34} j-s
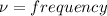
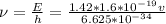
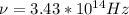
ii)wavelength is given as
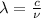
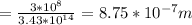
b) i) i)we know that
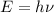
where E is energy
h = plank's constant = 6.625* 10^{-34} j-s
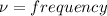
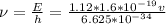
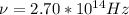
ii)wavelength is given as