Answer:
5953.42 J
Step-by-step explanation:
Given:
Initial volume,
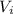 = 100 in³
= 100 in³
Final Volume,
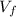 = 10 in³
= 10 in³
Initial pressure = 50 psia
Temperature = 100° F = 310.93 K
For isothermal reversible process, work done is given as:
Work done =
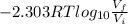
Where,
R is the ideal gas constant = 8.314 J/mol.K
or
Work done =
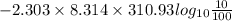
or
Work done = 5953.42 J