Answer:
a) 0,2% w/v
b) r=500
c) 0,0182 M
d) 0,0145 m
e) 0,0137 equivalents
Step-by-step explanation:
a) % w/v means mass of solute in grams per 100 mililiter of solution. Thus:
%w/v=
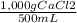 ×100 = 0,2%w/v
×100 = 0,2%w/v
b) Ratio strength is a way to express concentration. For w/v is in 1g of solute r mililiters of solution have. Thus, r = 500 because we have in the first 1 g of CaCl₂ in 500 mL of solution.
c) Molarity is moles of solute per liter of solution, thus:
1,000 g of CaCl₂ ×
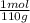 = 9,09×10⁻³ moles of CaCl₂
= 9,09×10⁻³ moles of CaCl₂
500 mL of solution ×
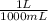 = 0,500 L of solution
= 0,500 L of solution
M =
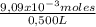 = 0,0182 M
= 0,0182 M
d) Molality is moles of solute per kg of solution.
Specific gravity is the ratio between density of the solution and density of a reference substance (Usually water). With a specific gravity of 0,8:
kg of solution = 0,500 L of solution ×
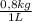 = 0,625 kg of solution
= 0,625 kg of solution
m =
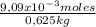 = 0,0145 m
= 0,0145 m
e) In a salt, equivalents are the number of moles ables to replace one mole of charge. In CaCl₂ is ¹/₂ because with ¹/₂ moles of CaCl₂ it is possible to replace 1 mole of charges. Thus, in 1,5 L there are:
1,5 L ×
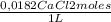 ×
×
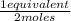 = 0,0137 equivalents
= 0,0137 equivalents
I hope it helps!