Answer:
The compound is
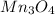
Step-by-step explanation:
The mass percentage of Mn is 72.1% and the mass percentage of O is 27.9%.
The mass percentage of a compound is given by:
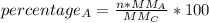
where:
n is its coefitient in the compund formula
MMa=Molar mass of the element A
MMc=Molar mass of the compound
So, we can figure out which compound is by dividing the percentage by its molar mass
Mn=72.1÷54.938045=1.31239
O=27.9÷15.9994=1.74382
Then, we divide each result by the smaller one (Mn)
Mn=1.31239÷1.31239=1
O=1.74382÷1.31239=1.3287
Each the realation of Mn:O is 1:1.3287
Then we multiply each result by 3:
Mn=1×3=3
O=1.3287×3=3.986≈4
Finally we figure out that the compound has 3 atoms of Mn and 4 atoms O. Result=
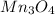
Mn3O4 is sometimes used as a starting material in the production of soft ferrites e.g. manganese zinc ferrite, and lithium manganese oxide, used in lithium batteries.