Step-by-step explanation:
It is given that 44.45 g of hydrocarbon gas produces 2649 kJ or
 of heat.
of heat.
Also here, 1 lb = 453.592 g.
Therefore, amount of energy released by 453.592 g of hydrocarbon gas will be calculated as follows.
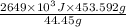
=

It is known that 1 J =
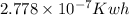 .
.
Hence,
 =
=

= 7.508 Kwh
Thus, we can conclude that the combustion of exactly one pound of this hydrocarbon gas produce 7.508 Kwh energy.